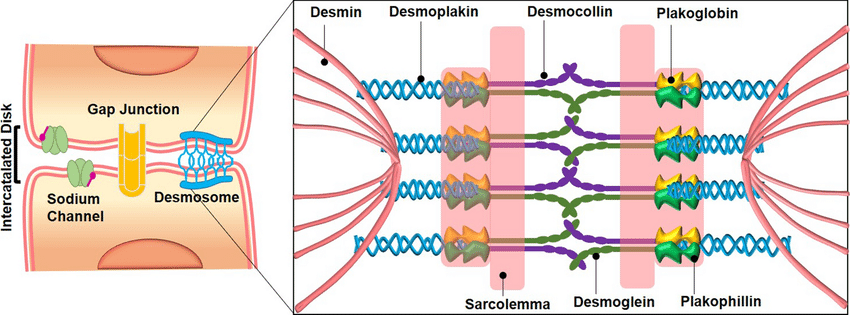
Desmosomes are adhesive protein complexes in the intercellular junction that help maintain the integrity of tissues. Desmosomes are mainly seen in tissues that are prone to mechanical stress. Eg. cardiac muscles, epithelial cells, neck of the uterus, etc.
Types of Desmosomes
There are three types of desmosomes- spot desmosomes, belt desmosomes, and hemidesmosomes.
Spot Desmosomes
- Spot desmosomes are also called Macula adherens. They hold epithelial cells at the points of contact and are thus called spot welds.
- They appear discoid and are made of non-glycosylated proteins like desmoplanktons I and II along with mucopolysaccharides.
- Intermediate keratin filaments called tonofilaments form a loop from the cytoplasm and back.
- Thinner filaments from the plaque transverse the plasma membrane forming trans-membrane linkers.
Belt Desmosomes
- These are present in columnar cells below the tight junctions.
- They appear as bands of girdle around the inner surface.
- This girdle is made of actin microfilaments and intermediate filaments.
- The former is contractile while the latter is structural.
- Here, the plasma membranes of connecting cells are ticker and parallel.
Hemidesmosomes
- Hemidesomsomes are half desmosomes that are similar to spot desmosomes.
- However, their basal surface joins the basal lamina.
- Their function is anchoring extracellular proteins to the cell. Eg. Collagen.
- Hemidesmosomes connect the extracellular matrix to the keratin cytoskeleton.
- They can maintain tissue integrity and dermo-epidermal adhesion.
- They also take part in intracellular signaling.
Desmosomes and Hemidesmosomes
- Desmosomes are the cell surface attachment site for intermediate filaments and hemidesmosomes are for the cell-substrate contacts.
- In each junction, the cytoplasmic domains of certain transmembrane junction components contain unusually long carboxy-terminal tails not found in those family members involved in the linkage of actin to the cell surface.
- The transmembrane molecules of the desmosome are calcium-dependent adhesion molecules, while those in the hemidesmosome are cell-matrix receptors from the integrin class.
- These domains are important to regulate the junction assembly and specific attachment of intermediate filaments with the help of associated adapter proteins.
- They are mainly structural components that maintain tissue integrity, they are also the acceptors and effectors of cell signaling pathways.
- Constituents of many desmosomes and hemidesmosomes are phosphoproteins.
- In some cases, they also regulate specific phosphorylation sites of protein-protein interactions.
- In addition, a more active role in transmitting signals that control morphogenesis during development and possibly even regulate cell growth and differentiation is being defined for cytoplasmic and membrane components of these junctions.
Function of Desmosomes
- Desmosomes are interconnections between cells to hold them together in tissues.
- They provide mechanical support to the tissues by distributing mechanical forces throughout the cells of tissues.
- Desmosomes function as elastic sheets that keep the tissues intact and at the same time make them flexible on their own without affecting the integrity of tissues.
- They play an important role in the transduction of intracellular signals which also helps with cell regulation and its behaviour.
References
- Nievers MG, Schaapveld RQ, Sonnenberg A. Biology and function of hemidesmosomes. Matrix Biol. 1999 Feb;18(1):5-17. doi: 10.1016/s0945-053x(98)00003-1. PMID: 10367727.
- Agarwal, P. V. |. V. (2004). Cell biology, Genetics, Molecular Biology, Evolution, and Ecology: Evolution and Ecology. S. Chand Publishing.
- https://www.cell.com/current-biology/fulltext/S0960-9822(11)00477-5
- Green KJ, Jones JC. Desmosomes and hemidesmosomes: structure and function of molecular components. FASEB J. 1996 Jun;10(8):871-881. doi: 10.1096/fasebj.10.8.8666164. PMID: 8666164.
Additional Reading
- Transport Mechanism Across Cell Membrane
- What is Phagocytosis and Pinocytosis?
- Structure and Function of Tight Junction
- Gap Junction Definition and Characteristics