One of the most significant contributions to the manipulative powers of modern biologists has been the development of cell and tissue culture techniques. Plant cell and tissue culture is fundamental to various aspects and scope of biotechnology and is of great interest to molecular biologists, plant breeders, and industrialists.
The crucial part of plant tissue culture is choosing the right media composition. Here, we will see more about plant tissue culture media composition used for successful propagation.
What is Plant Tissue Culture?
Tissue culture is the process where small pieces of living cultures are isolated from an organism and grown aseptically for indefinite periods on a semi-defined and defined nutrient media. The explants used for the cultures range from seedlings and organs to small single cells and protoplasts.
It was shown that plant tissue explants could proliferate by repeated cell divisions when suitable culture media was provided. This media usually contains auxins and cytokinins due to their effects on cell growth and divisions. Substances like coconut milk are also sometimes used since in-vivo it supports the growth and development of embryos in certain plants.
When appropriate culture conditions were provided, cell masses could proceed along various developmental pathways to regenerate shoot and root systems to grow into whole plants eventually.
Thus, the capacity of a single cell to regenerate its phenotype of a complete and differentiated organism from which it is derived is called totipotency.
Plant Tissue Culture Requirements
The most challenging requirement for in vitro plant tissue culture is to conduct the operations under aseptic conditions. Bacteria and fungi are the most common contaminants observed in the cultures. These microbes are omnipresent in our environment and on coming into contact with the culture media, find optimal conditions and grow much faster than the cultured tissue.
Consequently, the tissue is killed due to contamination. The contaminants may also give out metabolic waste which is toxic to the plant tissues. Because of this, the maintenance of a completely aseptic environment inside the culture vial where tissue growth is essential.
Plant Tissue Culture Equipment
The laboratory is the biggest equipment for tissue culture. A tissue culture lab should be prepared in such a manner that no microorganism or any type of contamination is possible. A standard tissue culture lab will have the following.
Media Room
It is here the culture media is prepared for the culture.
Culture Room
This room should be dust-free with automatic door closure for a no-contact entry and exit The room temperature should be maintained at 25 degrees and must be monitored using a thermometer and hydrometer for humidity levels. This room will also have special shelving to store the cultures, flasks, jars, Petri dishes, etc.
Inoculation Room
For aseptic work of the experimentation material, the inoculation room should be used in such a way that there should be no suffocation during work. Usually, a laminar airflow cabinet may be used for this.
Apparatus Used
- Autoclave
- Weighing balance
- pH meter
- Microscope
- Hot-air oven
- Glass materials
- Spirit lamp or Bunsen burner
- Forceps
- Needles
Chemicals
Commonly used chemicals for tissue culture include sodium dichromate to wash the glass wares, and other chemicals such as chromic acid, mercuric chloride, alcohol, etc.
Sterilization
Sterilization methods are used to sterilize the vessels and instruments used for tissue culture. Filtering, dry heat oven, autoclaving, etc are commonly used methods. The culture media is also sterilized. For these purposes, there is usually a sterilization room as well as a transfer area.
The process of sterilizing the plant material involves the following steps.
- Clean the plant material under running water.
- It is then treated with the detergent solution and later in 70% alcohol solution.
- The material is rinsed with distilled water.
- In the final stage, it is treated with sterilizing agents such as chlorine water, sodium hypochlorite, bleaching powder, or mercuric chloride.
Media Preparation
The culture media is the last but the most significant equipment involved. There are various types of culture media prepared based on the optimal growth of the plant parts used.
The composition of the media will be different for each plant type and the part used. The common ingredients are organic and inorganic nutrients, growth regulators, gelling agents, etc.
The media should be prepared with high-purity chemicals. Before starting, the preparation stock solution of micro and macro salts, vitamins, auxins, etc, should be prepared and stored in a refrigerator.
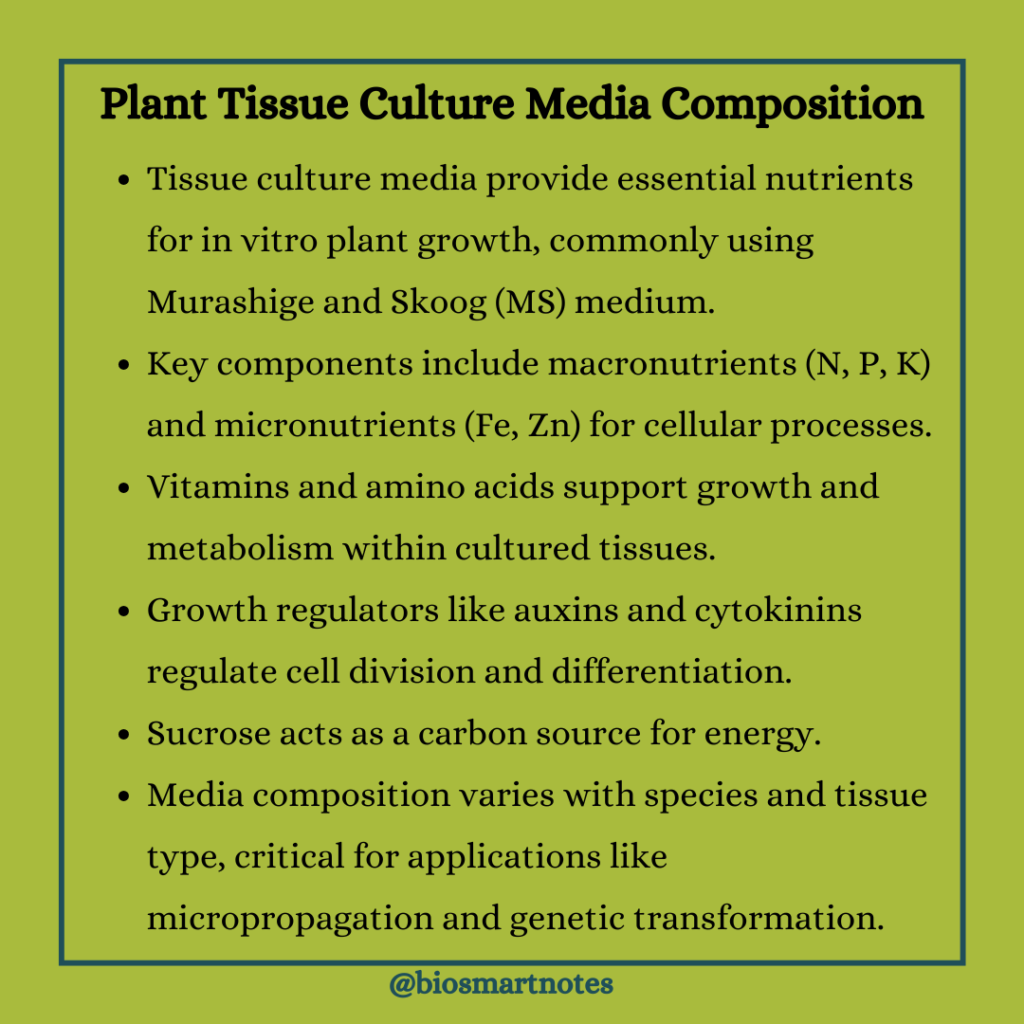
Plant Tissue Culture Media Composition
The plant tissue culture media is composed of various ingredients in the form of nutrients, growth regulators, gelling agents, etc. These ingredients are used in different proportions and variations depending upon the explant used for the procedure. The basic components of tissue culture media are
- Inorganic nutrients
- Carbon sources
- Organic supplements
- Growth regulators
- Solidifying agents
Inorganic Nutrients
The inorganic nutrients in plant tissue culture media include macronutrients such as nitrogen, phosphorous, calcium, potassium, magnesium, sulfur, and micronutrients such as iron, copper, boron, zinc, manganese, cobalt, molybdenum, etc.
Carbon and Energy Sources
Sucrose is the most preferred source of carbon in plant tissue culture. It supports uniform plant growth while fructose is less efficient. While autoclaving the medium, sucrose is converted into glucose and fructose. First glucose is used followed by fructose in the medium.
Apart from these, other energy sources used for tissue culture are lactose, galactose, raffinose, maltose, cellobiose, melibiose and trehalose generally yield inferior results.
Plant cells and tissues in the culture medium need external carbon sources for energy since they lack autotrophic ability. Even green tissues during the culture period are not autotrophs. The addition of external carbon sources to the medium initiates the proliferation of cells and the regeneration of green shoots.
Organic Supplements
Vitamins
Plants use vitamins as catalysts for different metabolic processes and they synthesize them endogenously. When plant cells and tissues are grown in vitro, some essential vitamins are synthesized but only in optimum quantities. Hence it is necessary to supply the medium with the required vitamins and amino acids to achieve the best growth of the tissues.
Thiamine (B1), nicotinic acid (B3), pyridoxine (B6), pantothenate, and myoinositol are commonly used. Among these thiamin is the basic vitamin required by cells and tissues.
Amino acids
Tissues that are cultured through plant tissue culture are generally capable of producing amino acids that are necessary for different metabolic processes. Despite this, the addition of amino acids into the media is essential for stimulating cell growth in protoplast cultures and for establishing cell lines.
Unlike inorganic nutrients, amino acids are taken up more rapidly by plant cells. Casein hydrolysate (0.5 to 0.1 %). L-glutamine, L-arginine, L-asparagine, L-cysteine, L-glycine, etc are common sources of organic nutrients used in culture media.
Other Organic Supplements
Culture media are often supplemented with a variety of organic extracts that have constituents of an undefined nature. They include proteins like casein, yeast, malt extract, hydrolase, coconut milk, ground banana, orange juice, tomato juice, etc.
In tissue culture, success requires the use of casein and hydrolases in 5-20% and 0.05-1% respectively. Similarly, potato extract is a suitable medium for anther culture. Generally, the use of natural extracts is avoided because the quantity of growth-promoting constituents varies with the age of the tissue from which the explant has been obtained thereby affecting the reproducibility of the results.
Activated Charcoal (AC)
The addition of activated charcoal stimulates the growth and differentiation of orchids, carrots, and tomatoes. It can also inhibit the growth of tobacco, soybeans, etc. The activated charcoal is acid-washed and neutralized before adding at a concentration of 0.5-0.3% into the media. It helps reduce toxicity by removing toxic compounds such as phenols from the media.
Antibiotics
Antibiotics retards cell tissue growth. Some plant cells will have infections due to microbes and the use of antibiotics prevents this. Streptomycin and kanamycin are commonly used in low concentrations. Its use does not inhibit the growth of the cell culture.
Growth Regulators
Mainly, there are four growth regulators for the plants- auxins, gibberellins, cytokinins, and ascorbic acid.
Auxin
There are different types of auxins such as,
- Indole-3 acetic acid (IAA)
- Naphthalene acetic acid (NAA)
- Indole-3-butyric acid (IBA)
- Naphthoxy acetic acid (NOA)
IAA occurs naturally in plants which induces cell division. Hormones of this group are involved in the elongation of stems, internodes, tropism, apical dominance, abscission, routine, etc.
Cytokinins
These are adenine derivatives concerned with cell division, modifications of apical dominance, and shoot differentiation. They can activate RNA synthesis and stimulate the activities of proteins and enzymes.
Gibberellins and Abscisic Acid (ABA)
These are required to enhance and inhibit growth. Gibberellins promote the growth of cell culture at low density and enhance callus growth. They can also induce dwarf or stunted plantlets to elongate. ABA on the other hand stimulates or inhibits callus growth, depending upon the species.
Solidifying Agents
Gels provide support to tissue growing in static conditions. Agar gels do not react with media constituents and are not digested by plant enzymes either. So they can remain stable at all feasible incubation temperatures.
Gelatin can be stable at a high concentration of 10% but melts at low temperatures. The pH of the media should be 5-6 before sterilization.
Preparation of Tissue Culture Media
Media preparation can be a time-consuming process. Media commonly used for tissue culture are available in the market as dry powders.
- The simplest method of preparing media is to dissolve the powder continuing inorganic and organic nutrients in a particular quantity of distilled water.
- After the mixture of the contents in water, sugar, agar, and other organic supplements are added as required.
- Finally, the volume is raised to 1 liter.
- The pH is adjusted and the media is autoclaved.
Powdered media are useful for the propagation of plant species that require nutrients according to the residue of standard media. For experiments in which a change in the quantity and quality of media constituents becomes necessary, it is desirable to weigh and dissolve each ingredient separately before mixing them.
Another convenient procedure is to prepare stalk solutions which when mixed in appropriate quantities constitute a basal medium.
Mistakes in the media preparation can do greater harm than any other fault in tissue culture technique. All steps in the media preparation and composition must be followed carefully.




