Vitamin E is a plant-based vitamin and is an important one for biological functions. Here you will see various properties of vitamin E.
Vitamin E was discovered as an active principle in 1920 independently by Evans and Mattill. Evans and his associates isolated two compounds with its activity from wheat germ oil, in 1936. They named it tocopherols. Later, five more tocopherols were discovered from other cereal grains. Since its deficiency causes sterility in animals, it is also called an anti-sterility factor.
Characteristics of Vitamin E
- Vitamin E or tocopherols are present in several plant oils, especially that of cereals, soybeans, and peanuts.
- It is absent in olive oil and fish liver oils.
- Vit E is a fat-soluble vitamin.
- It is composed of tocopherols and tocotrienols, both having four components each.
- There are four isomers of each of these, namely alpha, beta, gamma, and delta.
- Of these alpha tocopherol and gamma tocopherols are the most abundant forms.
- γ-Tocopherol is the only active form of this vitamin that can reverse the effects of its deficiency.
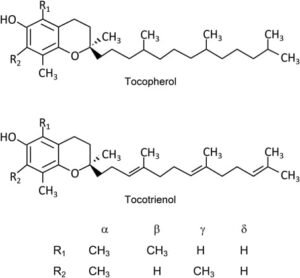
Structure of Vitamin E
Vitamin E has a molecular structure with a chromanol ring and a side chain at the C2 position.
- Vitamin E is a group of eight different compounds: α-, β-, γ-, and δ-tocopherols and their corresponding four tocotrienols.
- The four tocopherols consist of a saturated phytyl side chain.
- The tocotrienols have an unsaturated isoprenyl side chain.
- Side chains of tocotrienols have three double bonds at C3′, C7′, and C11′.
- The α-, β-, γ-, and δ-forms differ in the number and position of methyl groups on the chromanol ring.
- The α-forms of tocopherol and tocotrienol have three methyl groups at C5, C7, and C8 of the ring.
- The β- and γ-forms have two and the δ-forms have only one methyl group.
Properties of Vitamin E
- Vitamin E has a light yellow color and has an oily texture.
- This vitamin is found in the non-saponifiable part of vegetable oils.
- It is heat resistant and can stand up to 200 degrees Celsius.
- It is acid-resistant but vulnerable to alkalis, oxidized by UV rays.
Metabolism of Vitamin E
Excess amounts of alpha-tocopherols are converted into alpha CEHC and excreted through urine. The other forms of tocopherols also have similar metabolic pathways but the exact path is still unclear.
Biological Importance of Vitamin E
- Vitamin E is a natural antioxidant.
- Tocopherols help in the prevention of lipid oxidation and that of Vit A.
- It is a vital nutrient for growth and reproduction and works on the mesodermal tissues by preserving Vit A.
- Compounds such as phenols and other vitamins like vitamin C can enhance the efficiency of vitamin E as an antioxidant.
- A deficiency of vitamin E causes sterility in rats, muscular dystrophy in rabbits and guinea pigs, capillary damage in chicks due to creatinuria, encephalomalacia in poultry, and hemolytic anaemia in monkeys.
References
- Rizvi S, Raza ST, Ahmed F, Ahmad A, Abbas S, Mahdi F. The role of vitamin E in human health and some diseases. Sultan Qaboos Univ Med J. 2014 May;14(2):e157-65. Epub 2014 Apr 7. PMID: 24790736; PMCID: PMC3997530.
- CHAPTER 1: Vitamin E: Structure, Properties and Functions. Doi:
https://doi.org/10.1039/9781788016216-00001
Additional Reading
- Properties And Biological Role Of Vitamin A
- Properties and Biological Importance of Vitamin B
- Biological Role and Properties of Vitamin C
- Properties And Biological Role of Vitamin D