Fats and their derivatives are present in every living cell. Therefore, fat metabolism is an important process of every living cell.
They are important to protoplasm both as a fuel and structural component. In storage organs fats accumulate to such an extent as to make up 50% of dry weight. Like carbohydrates, fats also contain carbon, hydrogen, and oxygen but differ from the former in their molecular structure and in the fact that they have much less oxygen than carbon and hydrogen.
Fats can consume more oxygen and liberate more energy than carbohydrates. On complete oxidation, fats can liberate twice the energy when compared with carbohydrates. Due to this, carbohydrates are converted into fats when ripening seeds. Thus, together with carbohydrates and proteins, fats form an important trio of plant foods.
Solubility of Fats
Fats are insoluble in water and can be stored in cells in large amounts without disturbing their osmotic relation. They are especially abundant in seeds where they form an important food reserve. Lipids are soluble in organic solvents like chloroform, benzene, and ether.
Eg. Groundnut, walnut, cotton, sesame, castor, beans, coconut and palms. Olive oil is found in fruit tissues.
Classification of Lipids
Lipids are esters of fatty acids and alcohol. They are categorized into simple lipids, compound lipids and derived lipids.
Simple Lipids
Simple lipids are esters of monocarboxylic aliphatic acids and alcohol. It yields only this type of compound on hydrolysis.
The most abundant and physiologically important simple lipids are fats and waxes. In plants fat like lipoidal substances such as waxes, cutin, suberin, etc are also formed.
Compound Lipids
Compound lipids contain other groups such as phospholipids, phosphoric acid, and glycolipids which contain carbohydrates and lipoproteins with proteins.
Phospholipids are important constituents of cell membranes and subcellular structures derived from lipids are hydrolytic products of naturally occurring lipids and include fatty acids, alcohol, and steroids. They have higher molecular weight as well.
Fats are esters of high molecular weight fatty acids and glycerol. Glycerol is a trihydric alcohol and can react with three molecules of fatty acids to produce a fat molecule.
1 Glycerol + 3 Fatty acids —> Lipid
1 Glycerol + 3 Palmitic acid —> Tripalmitin
In a fat molecule, one, two, or all the three hydroxyl groups of glycerol may be combined with fatty acids forming mono, di, and triglycerides, respectively.
Types of Fats
Waxes
Waxes are esters of high molecular weight monohydric alcohols and high molecular weight fatty acids. A common wax seen is the poppy wax formed of cetyl alcohol and palmitic acid.
Waxes are usually harder than fats and do not hydrolyze as readily as fats. They are impervious to water and are characteristic of plants of dry habitat where they help to reduce cuticular transpiration.
Wax deposited in the form of grains or rods on the surface of leaves and fruits forms a covering called bloom. The bloom may be seen on the leaves of Agave americana and Calotropis, fruits of plums, peaches etc. The waxy bloom on the upper surface of leaves of floating plants prevents clogging of stomata.
Cutin
Cutin is formed in the outer walls of the epidermal cells of plants. Sometimes it forms a thick layer called the cuticle.
Suberin
Suberin is characteristic of the wall of cork cells. It is sometimes found on the walls of endodermal cells as well. These substances make cell walls almost impermeable to water.
Volatile Oils
Essential oils or volatile oils are also a type of fats. These aromatic oils are also called ethereal oils as they are readily soluble in ether. They are chemically different from fats and fatty oils.
Volatile oils do not have nutritional value and are generally considered waste products of plant metabolism. Lemon oil, clove oil, camphor, menthol, eucalyptus oil, etc are economically important volatile oils that are used in making perfumes and cosmetics, in the food industry, paints, varnishes, medicines, insect repellents, etc.
Types of Fatty Acids
- Fatty acids in fats are of two kinds, saturated and unsaturated. The unsaturated fatty acids have low melting points.
- Common saturated fatty acids are palmitic acid (C15H31COOH) and stearic acid (C17H33COOH).
- Common unsaturated fatty acids are Oleic acid (C17H20COOH), linoleic acid (C17H31COOH), and linolenic acid (C17H29COOH).
- The fatty acids of plants consist of an even number of carbon atoms. Eg. Palmitic acid is seen in palm oil, linolenic acid in linseed oil, etc.
- Vegetable fats are usually liquid at room temperature and are called oils because they are rich in unsaturated acids.
- Common fatty acids contain a minimum number of 16 C atoms in their molecules. Capric acid with 10 carbon atoms is an exception.
Processes of Fat Metabolism
Fat metabolism involves both synthesis and catabolism. Each process has several steps. Fat catabolism is also called oxidation which can be alpha oxidation and beta oxidation. Let’s find out more about these processes.
Fat Synthesis
Fats yield glycerol and fatty acids on hydrolysis. This process is called saponification. Saponification is a reversible reaction. It is generally believed that fats are synthesized in plants by condensation of one molecule of glycerol with three molecules of fatty acids under the influence of enzyme lipase.
Glycerol and fatty acids are derived by enzymatic action of carbohydrates through several intermediate steps. Synthesis of fat can be divided into the following phases,
- Synthesis of fatty acids
- Synthesis of glycerol
- Condensation of fatty acids and glycerol into fatty acids.
A Synthesis of Fatty Acids
There are two sites in the cell where fatty acids and fats are synthesized. The principal site is smooth endoplasmic reticulum and microsomes. The enzymes occur in soluble form in the cytoplasmic matrix. The second site is mitochondria.
Fatty acids participating in fat synthesis are long-chain compounds having 16 or more number of carbon atoms. These long-chain molecules are synthesized from a highly reactive 2-carbon compound, acetyl CoA (CH3CO-S CoA). This compound is produced as an intermediate in the respiratory breakdown of sugars and fats.
Synthesis takes place in a series of repeated stepwise reactions. In each step, the carbon chain is lengthened by the addition of acetyl CoA atoms, in the presence of an enzyme acetyl CoA carboxylase.
Enzyme Complexes
According to Green (1960), two enzyme complexes and five cofactors ( ATP, Mn++, Biotin, NADPH and CO2) are essential for the synthesis of fatty acids. Each step consists of two reactions which involve the two multi-enzyme complexes.
- One of the specific enzymes has the vitamin biotin as a prosthetic group and requires ATP as the supplier of energy for the activation of acetyl CoA
- Mn++ and carbon dioxide act as cofactors.
- Biotin combines with carbon dioxide to form a biotin-CO2 complex which attaches to enzymes.
- The second enzyme complex involves NADPH which supplies energy and brings about reduction.
Steps of Fatty Acid Synthesis
Step I
In the first reaction, CO2 combines with acetyl CoA to produce a highly reactive malonyl CoA which is the derivative of 3-carbon malonic acid.
Step II
In the second reaction, highly reactive malonyl CoA reacts with another molecule of acetyl-CoA in the presence of a specific enzyme called fatty acid synthetase and CoA NADPH2, resulting in the elimination of CO2. The resultant compound is a 4-carbon compound.
Step III
This 4-carbon compound undergoes a condensation reaction with another molecule of malonyl CoA by repetition of the same reaction sequence to give rise to a 6-carbon compound. This process is repeated until a long chain fatty acid (16-18 carbon chain) is produced.
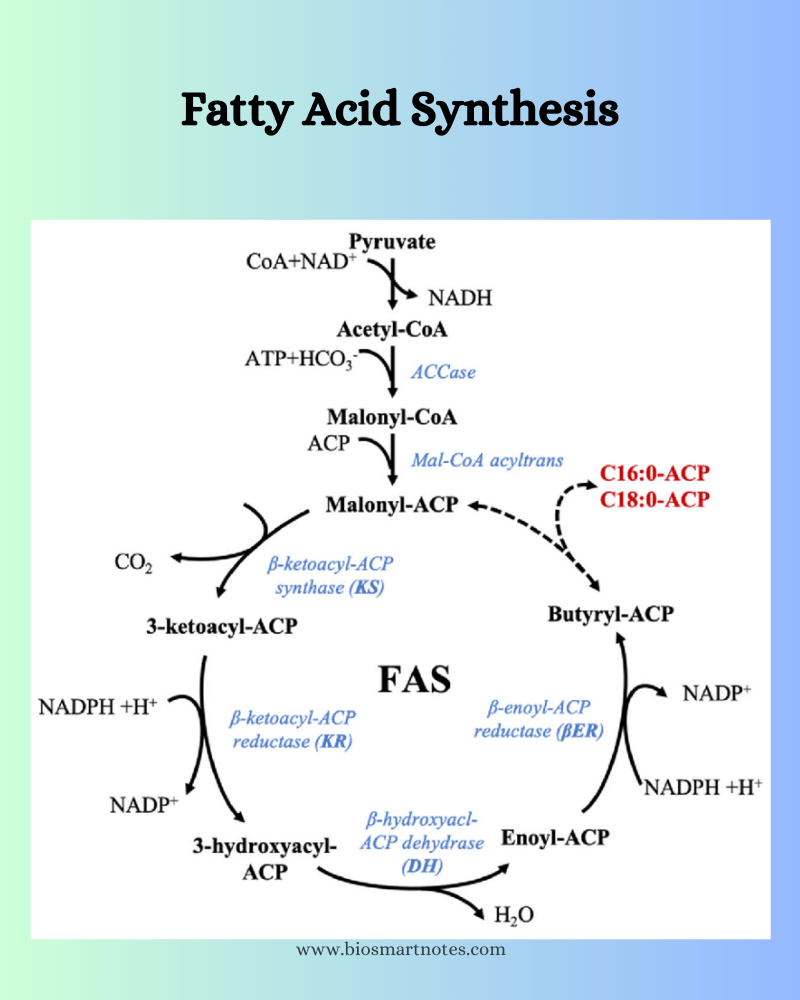
The enzyme fatty acid synthetase is a complex of many enzymes and an acyl carrier protein called ACP. The second reaction is a summary of many reactions, which can be categorized into three stages.
- Initiation reaction where the acetyl CoA transfers its acetyl group to one of the SH groups of the multienzyme complex of fatty acid synthetase.
- Chain elongation reaction has six different reactions involved.
- Chain elongation reaction starts with the transfer of the malonic group from malonyl CoA to the second SH group of the enzyme complex.
- Then the acetyl and malonyl groups undergo a condensation reaction with a simultaneous decarboxylation reaction of the malonyl group to form a 4-carbon unit.
- This 4-carbon unit undergoes reduction, dehydration, and one more reduction reaction to form a 4-carbon compound called butyryl CoA.
- Now, the fatty acid residue is transferred back to the initiation reaction to form a cycle for repeated malonyl transfer, until the fatty acid residue has 16-18 carbon atoms.
- The termination reaction starts when the fatty acid residue attains the desired length and the elongation process is stopped. Now, the cyclic reactions will come to an end and this fatty acid residue is utilized for fat synthesis. Once this process is complete, the enzyme becomes free for another set of reactions.
B Synthesis of Glycerol
Glycerol is synthesized from dihydroxyacetone phosphate (DHAP), produced as an intermediate in the glycolytic reaction of respiration. This compound is first enzymatically reduced to alpha glycerophosphate in the presence of NADPH2. The enzyme used here is alpha glycerophosphate dehydrogenase.
Dephosphorylation of alpha glycerophosphate (hydrolysed by phosphatase enzyme) results in its conversion to glycerol.
C Condensation of Fatty Acids and Glycerol
The final step of fat synthesis is the condensation of fatty acids and glycerol. There will be three fatty acid molecules for every molecule of glycerol.
Fatty acid first combines with acetyl CoA to produce an acyl CoA complex.
Fatty acid + Acetyl CoA —> Fatty acyl CoA complex
This complex combines with alpha glycerophosphate to form phosphatidic acid.
Now, it undergoes dephosphorylation in the presence of phosphatase to form a diglyceride.
The acylation of the free OH group of glyceride completes the biosynthesis of triglyceride or fat.
Oxidation Of Fatty Acids Or Catabolism Of Fatty Acids
Catabolism or breaking down of fatty acids can be through alpha-oxidation or beta-oxidation.
Alpha-Oxidation of Fatty Acids
Alpha-oxidation is a mechanism that is less important than beta-oxidation for the degradation of fatty acids.
- It is observed in cotyledons and young leaves and mainly occurs in the peroxisomes.
- This process occurs only in those branched fatty acids or fatty acids that contain a methyl group, which the mitochondria are unable to process through beta-oxidation.
- By this process, the long chain of fatty acids is gradually broken down until it is reduced to 12 carbon atoms.
- Fatty acids with less than 13 carbon atoms are not affected by this process.
- Moreover, alpha oxidation is not a complete breakdown of fatty acid as the resultant compounds will further undergo beta oxidation later in the mitochondria.
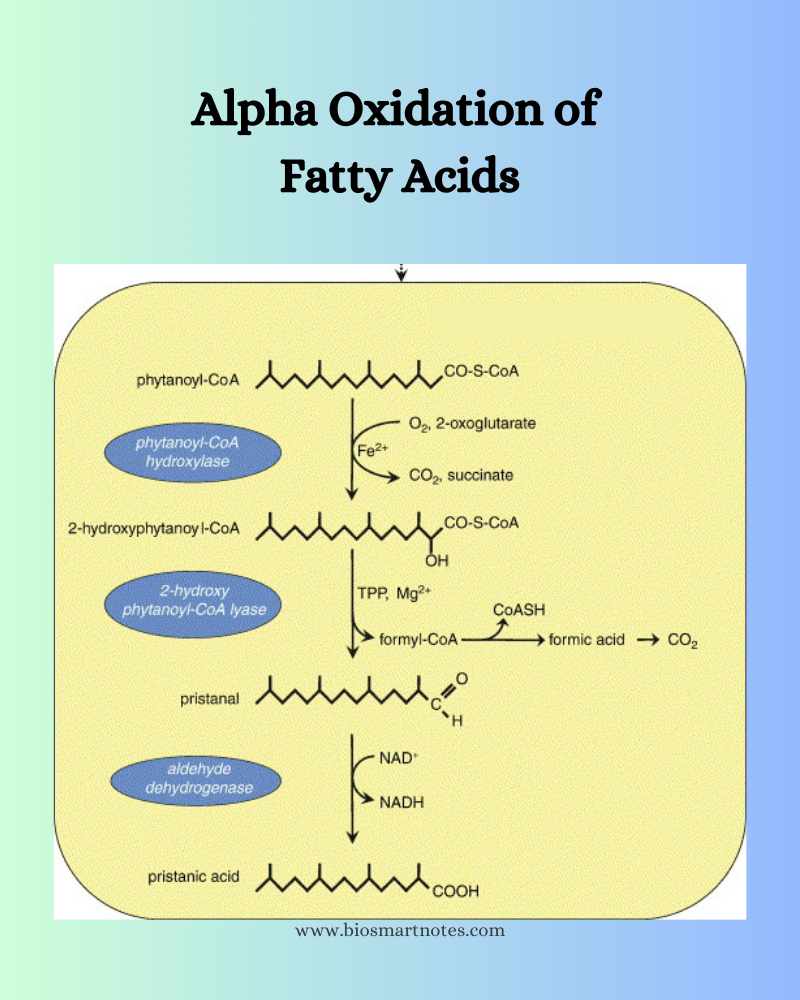
One complete alpha oxidation of fatty acids results in the elimination of one carbon in the form of CO2 from the COOH group. While the alpha carbon (which is adjacent to COOH) is oxidized. The alpha oxidation process takes place as follows.
Fatty acid is oxidatively decarboxylated in the presence of fatty acid peroxidase and hydrogen peroxide to form an aldehyde. In this process, CO2 is released from the COOH group and the alpha carbon atom is converted into an aldehyde group.
The aldehyde is further oxidized in the presence of aldehyde dehydrogenase to form a new fatty acid containing 1 carbon atom less than the original one. Here, NAD is reduced to NADH2.
The newly formed fatty acid will again be oxidized until it consists of 12 carbon atoms by the same process.
Beta Oxidation of Fatty Acids
Beta Oxidation is the chief process of fatty acid degradation in plants. This mechanism is well-established for saturated fatty acids.
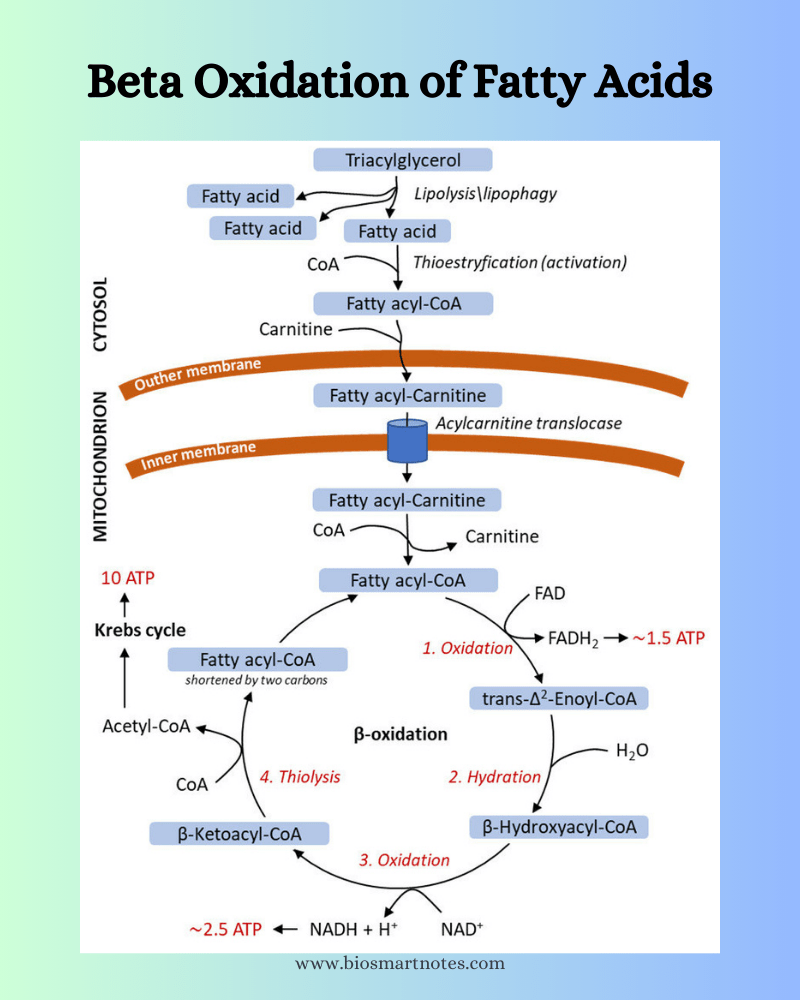
It involves the sequential removal of two carbon atoms in the form of acetyl CoA molecule from the carboxyl end of the fatty acid. This is called beta-oxidation because the beta-carbon of fatty acid is oxidized during this process to generate the carbonyl group.
- All the enzymes related to the beta-oxidation system are located on the inner membrane of the mitochondria.
- Though fatty acids are produced in the cytosol, beta-oxidation takes place in mitochondria.
- The mitochondrial trifunctional protein facilitates the beta-oxidation.
- The aim of beta-oxidation is the breaking down of fatty acids to generate acetyl CoA which will enter the TCA cycle and also produce the coenzymes NADH and FADH2.
References
- Remize,M.;Brunel,Y.;Silva,J.L.;Berthon,J.‐Y.;Filaire,E.Microalgaen‐3PUFAsProductionandUseinFoodandFeedIndustries.Mar.Drugs2021,19,113.https://doi.org/10.3390/md19020113
- What is the Difference Between Alpha and Beta Oxidation?
- Jansen, G. A., & Wanders, R. J. (2006). Alpha-Oxidation. Biochimica et Biophysica Acta (BBA) – Molecular Cell Research, 1763(12), 1403-1412. https://doi.org/10.1016/j.bbamcr.2006.07.012




